Chemistry compatible with AP*
The AP exams are tough, but imagine how much easier they'd be if you had the world's best AP Chemistry teachers teaching you the fundamentals and advanced concepts of chemistry. Learn the ins and outs of AP Chemistry from award-winning chemistry professors Gordon Yee and Dean Harman.
They'll teach you the powerful ideas behind chemistry that will help you score a perfect 5 on the AP Chemistry exam. Thinkwell's online AP Chemistry subscription gives you the AP Chemistry help you want through a complete web-based solution that includes all of the content you'll need to succeed in the classroom portion of AP Chemistry. Thinkwell's AP Chemistry study aid can be used in conjunction with your school's AP Chemistry class. The videos and exercises are available 24/7 and for one set price, so it's better than a tutor. Though similar to our college-level Chemistry course, AP Chemistry includes diagnostic assessments geared towards AP exam preparation.
Our complete Chemistry compatible with AP* package includes:- 12-month Online Subscription to our complete AP Chemistry course with video lessons, automatically graded AP Chemistry problems, and much more.
- Printed Notes (optional) are the AP Chemistry course notes from the Online Subscription printed in a black & white, on-the-go format. These are available for purchase from the AP Chemistry Course Site.
Printed Notes require the purchase of an online subscription.
(*AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, this product.)
Money-Back Guarantee
Chemistry compatible with AP* Materials
Online Subscription, 12-month access
Access to a complete online package that includes everything you need.
- Thinkwell's video lectures cover the comprehensive scope and sequence of topics covered on the AP Chemistry exam.
- Diagnostic tests that tell you exactly what you need to study.
- Our automatically graded exercises with immediate feedback allow you to quickly determine which areas you'll need to spend extra time studying.
- Concise, illustrated review notes help you distill the fundamental ideas, concepts, and formulas you'll need to know in order to succeed on the AP Chemistry exam.
- Subscriptions start when you are ready. Buy now and activate your course anytime you like. Wait up to one year to activate your subscription; your 12-month subscription doesn't begin until you say so!
Chemistry compatible with AP* Details
Thinkwell's AP Chemistry includes all of these features to prepare you for the big exam:
- Equivalent to 11th- or 12th-grade AP Chemistry
- More than 260 video lessons (see sample)
- 1000+ interactive AP chemistry problems with immediate feedback allow you to track your progress (see sample)
- Diagnostic tests that tell you exactly what you need to study
- AP Chemistry practice chapter tests for all 22 chapters, as well as a final exam to make sure you're ready for the AP Chemistry exam (only available in the homeschool version)
- Printable illustrated notes for each topic
- Closed captioning for all videos
- Glossary of more than 850 chemistry terms
- Engaging content to help students advance their knowledge of chemistry:
- Atoms, molecules, and ions
- Stoichiometry and the mole
- Reactions in aqueous solutions
- Gases, liquids, and solids
- Modern atomic theory and quantum mechanics
- Electron configurations and periodicity
- Chemical bonding and reactions
- Properties of solutions
- Acids and bases
- Thermochemistry, electrochemistry, and biochemistry
Table of Contents
(Expand All - Close All)1. An Introduction to Matter and Measurement
- 1.1 An Introduction to Chemistry and the Scientific Method
- 1.1.1 An Introduction to Chemistry
- 1.1.2 The Scientific Method
- 1.2 Properties of Matter
- 1.2.1 States of Matter
- 1.2.2 A Word About Laboratory Safety
- 1.2.3 CIA Demonstration: Differences in Density Due to Temperature
- 1.2.4 Properties of Matter
- 1.3 Scientific Measurement
- 1.3.1 The Measurement of Matter
- 1.3.2 Precision and Accuracy
- 1.3.3 CIA Demonstration: Precision and Accuracy with Glassware
- 1.3.4 Significant Figures
- 1.3.5 Dimensional Analysis
- 1.4 Mathematics of Chemistry
- 1.4.1 Scientific (Exponential) Notation
- 1.4.2 Common Mathematical Functions
2. Atoms, Molecules, and Ions
- 2.1 Early Atomic Theory
- 2.1.1 Early Discoveries and the Atom
- 2.1.2 Understanding Electrons
- 2.1.3 Understanding the Nucleus
- 2.2 Atomic Structure
- 2.2.1 Mass Spectrometry: Determining Atomic Masses
- 2.2.2 Examining Atomic Structure
- 2.2.3 CIA Demonstration: Flame Colors
- 2.3 The Periodic Table
- 2.3.1 Creating the Periodic Table
- 2.4 Chemical Nomenclature
- 2.4.1 Describing Chemical Formulas
- 2.4.2 Naming Chemical Compounds
- 2.4.3 Organic Nomenclature
3. Stoichiometry
- 3.1 Chemical Equations
- 3.1.1 An Introduction to Chemical Reactions and Equations
- 3.1.2 CIA Demonstration: Magnesium and Dry Ice
- 3.1.3 Balancing Chemical Equations
- 3.2 The Mole
- 3.2.1 The Mole and Avogadro's Number
- 3.2.2 Introducing Conversions of Masses, Moles, and Number of Particles
- 3.3 Solving Problems Involving Mass/Mole Relationships
- 3.3.1 Finding Empirical and Molecular Formulas
- 3.3.2 Stoichiometry and Chemical Equations
- 3.3.3 Finding Limiting Reagents
- 3.3.4 CIA Demonstration: Self-Inflating Hydrogen Balloons
- 3.3.5 Theoretical Yield and Percent Yield
- 3.3.6 A Problem Using the Combined Concepts of Stoichiometry
4. Reactions in Aqueous Solutions
- 4.1 An Introduction to Solutions
- 4.1.1 Properties of Solutions
- 4.1.2 CIA Demonstration: The Electric Pickle
- 4.1.3 Concentrations of Solutions
- 4.1.4 Factors Determining Solubility
- 4.2 Reactions Involving Solutions
- 4.2.1 Precipitation Reactions
- 4.2.2 Acid-Base Reactions
- 4.2.3 Oxidation-Reduction Reactions
- 4.3 Stoichiometry Problems in Solutions
- 4.3.1 Acid-Base Titrations
- 4.3.2 Solving Titration Problems
- 4.3.3 Gravimetric Analysis
5. Gases
- 5.1 Gases and Gas Laws
- 5.1.1 Properties of Gases
- 5.1.2 Boyle's Law
- 5.1.3 Charles's Law
- 5.1.4 The Combined Gas Law
- 5.1.5 Avogadro's Law
- 5.1.6 CIA Demonstration: The Potato Cannon
- 5.2 The Ideal Gas Law and Kinetic-Molecular Theory of Gases
- 5.2.1 The Ideal Gas Law
- 5.2.2 Partial Pressure and Dalton's Law
- 5.2.3 Applications of the Gas Laws
- 5.2.4 The Kinetic-Molecular Theory of Gases
- 5.2.5 CIA Demonstration: The Ammonia Fountain
- 5.3 Molecular Motion of Gases
- 5.3.1 Molecular Speeds
- 5.3.2 Effusion and Diffusion
- 5.4 Behavior of Real Gases
- 5.4.1 Comparing Real and Ideal Gases
6. Thermochemistry
- 6.1 An Introduction to Energy
- 6.1.1 The Nature of Energy
- 6.1.2 Energy, Calories, and Nutrition
- 6.1.3 The First Law of Thermodynamics
- 6.1.4 Work
- 6.1.5 Heat
- 6.1.6 CIA Demonstration: Cool Fire
- 6.2 Enthalpy
- 6.2.1 Heats of Reaction: Enthalpy
- 6.2.2 CIA Demonstration: The Thermite Reaction
- 6.3 Calorimetry
- 6.3.1 Constant Pressure Calorimetry
- 6.3.2 Bomb Calorimetry (Constant Volume)
- 6.4 Hess's Law and Enthalpies of Formation
- 6.4.1 Hess's Law
- 6.4.2 Enthalpies of Formation
7. Modern Atomic Theory
- 7.1 Electromagnetic Radiation and the Idea of Quantum
- 7.1.1 The Wave Nature of Light
- 7.1.2 Absorption and Emission
- 7.1.3 CIA Demonstration: Luminol
- 7.1.4 The Ultraviolet Catastrophe
- 7.1.5 The Photoelectric Effect
- 7.1.6 The Bohr Model
- 7.1.7 The Heisenberg Uncertainty Principle
- 7.2 Quantum Mechanics
- 7.2.1 The Wave Nature of Matter
- 7.2.2 Radial Solutions to the Schrödinger Equation
- 7.2.3 Angular Solutions to the Schrödinger Equation
- 7.3 Atomic Orbitals
- 7.3.1 Atomic Orbital Size
- 7.3.2 Atomic Orbital Shapes and Quantum Numbers
- 7.3.3 Atomic Orbital Energy
8. Electron Configurations and Periodicity
- 8.1 Electron Spin and the Pauli Exclusion Principle
- 8.1.1 Understanding Electron Spin
- 8.1.2 Electron Shielding
- 8.1.3 Electron Configurations Through Neon
- 8.1.4 Electron Configurations Beyond Neon
- 8.1.5 Periodic Relationships
- 8.2 Periodicity
- 8.2.1 Periods and Atomic Size
- 8.2.2 Ionization Energy
- 8.2.3 Electron Affinity
- 8.2.4 An Introduction to Electronegativity
- 8.3 Group Trends
- 8.3.1 Hydrogen, Alkali Metals, and Alkaline Earth Metals
- 8.3.2 Transition Metals and Nonmetals
9. Chemical Bonding: Fundamental Concepts
- 9.1 Valence Electrons and Chemical Bonding
- 9.1.1 Valence Electrons and Chemical Bonding
- 9.1.2 Ionic Bonds
- 9.1.3 CIA Demonstration: Conductivity Apparatus-Ionic versus Covalent Bonds
- 9.2 Lewis Dot Structures
- 9.2.1 Lewis Dot Structures for Covalent Bonds
- 9.2.2 Predicting Lewis Dot Structures
- 9.3 Resonance Structures and Formal Charge
- 9.3.1 Resonance Structures
- 9.3.2 Formal Charge
- 9.3.3 Electronegativity, Formal Charge, and Resonance
- 9.4 Bond Properties
- 9.4.1 Bond Properties
- 9.4.2 Using Bond Dissociation Energies
10. Molecular Geometry and Bonding Theory
- 10.1 Molecular Geometry and the VSEPR Theory
- 10.1.1 Valence-Shell Electron-Pair Repulsion Theory
- 10.1.2 Molecular Shapes for Steric Numbers 2-4
- 10.1.3 Molecular Shapes for Steric Numbers 5 & 6
- 10.1.4 Predicting Molecular Characteristics Using VSEPR Theory
- 10.2 Valence Bond Theory and Molecular Orbital Theory
- 10.2.1 Valence Bond Theory
- 10.2.2 An Introduction to Hybrid Orbitals
- 10.2.3 Pi Bonds
- 10.2.4 Molecular Orbital Theory
- 10.2.5 Applications of the Molecular Orbital Theory
- 10.2.6 Beyond Homonuclear Diatomics
- 10.2.7 CIA Demonstration: The Paramagnetism of Oxygen
11. Oxidation-Reduction Reactions
- 11.1 Looking In-Depth at Redox Reactions
- 11.1.1 Oxidation Numbers
- 11.1.2 Balancing Redox Reactions by the Oxidation Number Method
- 11.1.3 Balancing Redox Reactions Using the Half-Reaction Method
- 11.1.4 The Activity Series of the Elements
- 11.1.5 CIA Demonstration: The Reaction Between Al and Br2
12. Condensed Phases: Liquids and Solids
- 12.1 Intermolecular Forces
- 12.1.1 An Introduction to Intermolecular Forces and States of Matter
- 12.1.2 Intermolecular Forces
- 12.2 Physical Properties of Liquids
- 12.2.1 Properties of Liquids
- 12.2.2 CIA Demonstration: Boiling Water at Reduced Pressure
- 12.2.3 Vapor Pressure and Boiling Point
- 12.2.4 Molecular Structure and Boiling Point
- 12.2.5 Phase Diagrams
- 12.2.6 CIA Demonstration: Boiling Water in a Paper Cup
- 12.3 Solid State: Structure and Bonding
- 12.3.1 Types of Solids
- 12.3.2 CIA Demonstration: The Conductivity of Molten Salts
- 12.3.3 Crystal Structure
- 12.3.4 Calculating Atomic Mass and Radius from a Unit Cell
- 12.3.5 Crystal Packing
13. Physical Properties of Solutions
- 13.1 Characterizing Solutions
- 13.1.1 Types of Solutions
- 13.1.2 Molarity and the Mole Fraction
- 13.1.3 Molality
- 13.1.4 Energy and the Solution Process
- 13.2 Effects of Temperature and Pressure on Solubility
- 13.2.1 Temperature Change and Solubility
- 13.2.2 Extractions
- 13.2.3 Pressure Change and Solubility
- 13.3 Colligative Properties
- 13.3.1 Vapor Pressure Lowering
- 13.3.2 Boiling Point Elevation and Freezing Point Depression
- 13.3.3 Boiling Point Elevation Problem
- 13.3.4 Osmosis
- 13.3.5 Colligative Properties of Ionic Solutions
14. Chemical Kinetics
- 14.1 Reaction Rates
- 14.1.1 An Introduction to Reaction Rates
- 14.1.2 Rate Laws: How the Reaction Rate Depends on Concentration
- 14.1.3 Determining the Form of a Rate Law
- 14.2 Orders of Reaction
- 14.2.1 First-Order Reactions
- 14.2.2 Second-Order Reactions
- 14.2.3 A Kinetics Problem
- 14.3 Temperature and Rates
- 14.3.1 The Collision Model
- 14.3.2 The Arrhenius Equation
- 14.3.3 Using the Arrhenius Equation
- 14.4 Reaction Mechanisms
- 14.4.1 Defining the Molecularity of a Reaction
- 14.4.2 Determining the Rate Laws of Elementary Reactions
- 14.4.3 Calculating the Rate Laws of Multi-step Reactions
- 14.4.4 Steady State Kinetics
- 14.5 Catalysts
- 14.5.1 Catalysts and Types of Catalysts
- 14.5.2 A Word About Laboratory Safety
- 14.5.3 CIA Demonstration: Elephant Snot
- 14.5.4 CIA Demonstration: The Cobalt(II)-Catalyzed Reaction of Potassium Sodium Tartrate
- 14.5.5 CIA Demonstration: The Copper-Catalyzed Decomposition of Acetone
15. Chemical Equilibrium
- 15.1 Principles of Chemical Equilibrium
- 15.1.1 The Concept of Equilibrium
- 15.1.2 The Law of Mass Action and Types of Equilibrium
- 15.1.3 Converting Between Kc and Kp
- 15.2 Using Equilibrium Constants
- 15.2.1 Approaching Chemical Equilibrium
- 15.2.2 Predicting the Direction of a Reaction
- 15.2.3 Strategies for Solving Equilibrium Problems
- 15.2.4 Solving Problems Far from Equilibrium
- 15.2.5 An Equilibrium Problem Using the Quadratic Equation
- 15.3 Shifting Chemical Equilibrium
- 15.3.1 Le Châtelier's Principle
- 15.3.2 The Effect of Changing Amounts on Equilibrium
- 15.3.3 The Effects of Pressure and Volume on Equilibrium
- 15.3.4 The Effects of Temperature and Catalysts on Equilibrium
- 15.3.5 CIA Demonstration: NO2/N2O4
- 15.3.6 CIA Demonstration: Shifting the Equilibrium of FeSCN2+
16. Acids and Bases
- 16.1 Acid-Base Concepts
- 16.1.1 Arrhenius/Brønsted-Lowry Definitions of Acids and Bases
- 16.1.2 Hydronium, Hydroxide, and the pH Scale
- 16.2 Acid and Base Strengths
- 16.2.1 Strong Acids and Bases
- 16.2.2 CIA Demonstration: Natural Acid-Base Indicators
- 16.2.3 Weak Acids
- 16.2.4 Weak Bases
- 16.2.5 Lewis Acids and Bases
- 16.2.6 Trends in Acid and Base Strengths
17. Equilibrium in Aqueous Solution
- 17.1 Reactions of Acids and Bases
- 17.1.1 Strong Acid-Strong Base and Weak Acid-Strong Base Reactions
- 17.1.2 Strong Acid-Weak Base and Weak Acid-Weak Base Reactions
- 17.1.3 The Common Ion Effect
- 17.2 Buffers
- 17.2.1 An Introduction to Buffers
- 17.2.2 CIA Demonstration: Buffers in Action
- 17.2.3 Acidic Buffers
- 17.2.4 Basic Buffers
- 17.2.5 The Henderson-Hasselbalch Equation
- 17.3 Acid-Base Titration
- 17.3.1 Strong Acid-Strong Base Titration
- 17.3.2 CIA Demonstration: Barium Hydroxide-Sulfuric Acid Titration
- 17.3.3 Weak Acid-Strong Base Titration
- 17.3.4 Polyprotic Acid-Strong Base Titration
- 17.3.5 Weak Base-Strong Acid Titration
- 17.3.6 Acid-Base Indicators
- 17.4 Solubility Equilibria
- 17.4.1 The Solubility Product Constant
- 17.4.2 CIA Demonstration: Silver Chloride and Ammonia
- 17.4.3 Solubility and the Common Ion Effect
- 17.4.4 Fractional Precipitation
18. Thermodynamics
- 18.1 An Introduction to Thermodynamics
- 18.1.1 Spontaneous Processes
- 18.2 Entropy
- 18.2.1 Entropy and the Second Law of Thermodynamics
- 18.2.2 Entropy and Temperature
- 18.3 Gibbs Free Energy and Free Energy Change
- 18.3.1 Gibbs Free Energy
- 18.3.2 Standard Free Energy Changes of Formation
- 18.4 Using Free Energy
- 18.4.1 Enthalpy and Entropy Contributions to K
- 18.4.2 The Temperature Dependence of K
- 18.4.3 Free Energy Away from Equilibrium
19. Electrochemistry
- 19.1 Principles of Electrochemistry
- 19.1.1 Reviewing Oxidation-Reduction Reactions
- 19.2 Galvanic Cells
- 19.2.1 Electrochemical Cells
- 19.2.2 Electromotive Force
- 19.2.3 Standard Reduction Potentials
- 19.2.4 Using Standard Reduction Potentials
- 19.2.5 The Nernst Equation
- 19.2.6 Electrochemical Determinants of Equilibria
- 19.3 Batteries
- 19.3.1 Batteries
- 19.3.2 CIA Demonstration: The Fruit-Powered Clock
- 19.4 Corrosion
- 19.4.1 Corrosion and the Prevention of Corrosion
- 19.5 Electrolysis and Electrolytic Cells
- 19.5.1 Electrolytic Cells
- 19.5.2 The Stoichiometry of Electrolysis
20. Nuclear Chemistry
- 20.1 Radioactivity
- 20.1.1 The Nature of Radioactivity
- 20.1.2 The Stability of Atomic Nuclei
- 20.1.3 Binding Energy
- 20.2 Rates of Disintegration
- 20.2.1 Rates of Disintegration Reactions
- 20.2.2 Radiochemical Dating
- 20.3 Nuclear Fission and Fusion
- 20.3.1 Nuclear Fission
- 20.3.2 Nuclear Fusion
- 20.3.3 Applications of Nuclear Chemistry
21. Chemistry of Metals
- 21.1 An Introduction to Metals
- 21.1.1 Metallurgical Processes
- 21.1.2 The Band Theory of Conductivity
- 21.1.3 Intrinsic Semiconductors
- 21.1.4 Doped Semiconductors
- 21.2 Physical and Chemical Processes of Metals
- 21.2.1 The Alkali Metals
- 21.2.2 The Alkaline Earth Metals
- 21.2.3 Aluminum
- 21.2.4 CIA Demonstration: The Reaction Between Al and Br2
22. Nonmetals
- 22.1 An Introduction to Nonmetals and Hydrogen
- 22.1.1 General Properties of Nonmetals
- 22.1.2 Hydrogen
- 22.2 Group 14: Carbon and Silicon
- 22.2.1 General Properties of Carbon
- 22.2.2 Silicon
- 22.3 Group 15: Nitrogen and Phosphorus
- 22.3.1 Nitrogen
- 22.3.2 Phosphorus
- 22.4 Group 16: Oxygen and Sulfur
- 22.4.1 Oxygen
- 22.4.2 CIA Demonstration: Creating Acid Rain
- 22.4.3 Sulfur
- 22.5 Group 17: The Halogens
- 22.5.1 Halogens
- 22.5.2 Aqueous Halogen Compounds
- 22.6 Group 18: The Noble Gases
- 22.6.1 Properties of Noble Gases
23. Instructional Laboratory Demonstrations
- 23.1 Laboratory Techniques
- 23.1.1 CIA Demonstration: Laboratory Safety
- 23.1.2 CIA Demonstration: Chromatography
- 23.1.3 CIA Demonstration: Distillation
- 23.1.4 CIA Demonstration: Pipetting
- 23.1.5 CIA Demonstration: Dilutions
- 23.1.6 CIA Demonstration: Titrations
- 23.1.7 CIA Demonstration: Extractions
- 23.1.8 CIA Demonstration: Filtrations
- 23.1.9 CIA Demonstration: Weighing on an Analytical Balance
- 23.1.10 CIA Demonstration: Recrystallization
About the Author
Sample Video Lessons
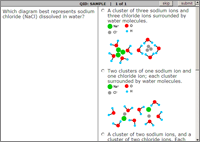
Sample Interactive Exercises